Products
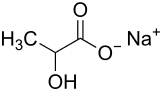
Sodium lactate
Sodium lactate is the salt of lactic acid with sodium hydroxide. The substance is available in liquid and solid form. The syrupy liquid is almost colorless and almost odorless and tasteless. The powder is miscible with water (>1.5g/ml).
In this product sector we work together with the european manufacturers Jungbunzlauer, Corbion / Purac (Purac ® ) and Galactic (Galacit ™).
1. Production
The main raw material for the production of sodium lactate is lactic acid. There are two established systems for industrial production: chemical production or by means of fermentation (biological).
Chemical production uses coal, petroleum products, or natural gas as feedstock. Although there are many chemical ways to produce lactic acid, the so-called acrylonitrile process has become established. Here, the starting products hydrogen cyanide and acetaldehyde are combined under high pressure and with the aid of a basic catalyst to form lactonitrile. After a purification step, sulfuric acid is added to the lactonitrile obtained to obtain lactic acid and ammonium salts by hydrolysis. The finished lactic acid is thus obtained via several distillation and purification steps. It is important to note that this chemical synthesis always yields a racemate, which must be further separated into the individual isomers if required.
Over 90% of the world's lactic acid is now produced by fermentation. The main starting material is glucose, which is obtained from corn or starch-containing plants (wheat, barley, potatoes, sugar cane, etc.). Here, the higher the starting materials, the higher the final product: high purity is obtained, for example, with sucrose from sugar cane or sugar beets. With a high purity content, the subsequent downstream processing steps are simpler and therefore more cost-effective. The actual production is then usually carried out in a so-called batch process: in simple terms, microorganisms are added to a glucose solution in a large container. Under specific reaction parameters, such as temperature, the microorganisms convert the glucose into ethanol, citric acid and lactic acid. Microorganisms here usually simply mean bacteria or fungal species. Classic bacterial strains are: Lb. Lactis BME5-18M, B. coagulans LA204, Bacillus sp. strain, and others, with average productivity varying from 0.25-2.5 g/l/h. The yield also changes from 36-97% depending on the bacterial strain and starting product. If the lactic acid is produced by fermentation, racemates are also obtained, but they contain a very high proportion of one isomer. In the mixture obtained, the lactic acid must now be separated by processes: Precipitation, filtration, evaporation, and crystallization are just a few processes to be mentioned here. The number of processing steps has a strong influence on the quality and price of the product. Despite the many processing steps, production by fermentation is simpler and thus more costly than chemical production.
The finished lactic acid is now mixed with sodium hydroxide solution, which serves as a base. This chemical reaction, also called redox reaction or neutralization, leads to the salt formation of the two reactants. The end products are the sodium lacatate salt and water.
2. Use/effect
Due to its biological compatibility and harmlessness, it is often used in food technology, medicine and cosmetics industry.
Since sodium lactate from the manufacturing process usually does not contain animal proteins, it can be taken without any problems in case of lactic acid intolerance. In food, the substance also goes by the E number 325. The food additive can be added to the product in "sufficient quantity" and is also considered safe in organic products.
Due to its chemical properties, the salt of lactic acid is used as an acidity regulator and humectant. In addition, sodium lactate influences the swelling capacity of protein: fats and water are better bound to the amino acids, thus reducing the leakage of these substances. Here, too, the products remain fresh and attractive to the customer for longer. This is also referred to as so-called melting salts or firming agents.
Similar to the food industry, the salt of lactic acid also serves as a humectant and buffer in the pharmaceutical industry.
In creams or ointments, together with other acids, the pH value can be precisely adjusted. Together with other substances, it replicates the skin's own Natural Moisturizing Factor (NMF). The skin is kept moist and protected by these hydrating substances. It is not surprising that large quantities are used in the cosmetics industry for similar purposes.
Sodium lactate is produced in biological processes as an intermediate or degradation product. It is a product that is thus biodegradable and does not harm the ecology.
Medicine uses sodium lactate to treat drug-induced arrhythmias: these include class I antiarrythmic drugs and pressor sympathomimetics. It is on the World Health Organization's list of essential medicines, the safest and most effective drugs needed in a health care system.
3. Further properties/facts
Sodium lactate, together with lactic acid, provides a physiological protective acid mantle on the skin. This acid protection (pH of 5.5) ensures controlled colonization of the skin with bacteria. If this protection is destroyed by too frequent washing, unwanted damage to the skin may occur.
Product details
| Qualities: | 50%, 50% pH 5.5, 60% |
|---|---|
| Appearance: | clear to white |
| CAS number: | 72-17-3 |
| Origin country: | Netherlands, Belgium, France |
| Physical state: | liquid |
| Container: | Canister, drum, IBC |
| INCI: | Sodium Lactate |
| Durability: | 2 years |
| Storage: | should be stored at a cool, dry and light shielded place, in sealed packaging |
More Products
Your contact

Manuel Bahr

Christine Ademes